Mercury's Atomic Emission Spectrum Is Shown Below
Recall that the atomic emission spectrum of hydrogen had spectral. Different types of.

Modelled Emission Spectra Of Atomic Mercury At Different Electronic Download Scientific Diagram
Neon signs or other gas discharge tubes as used for advertising sodium or mercury vapour lamps show atomic emission.

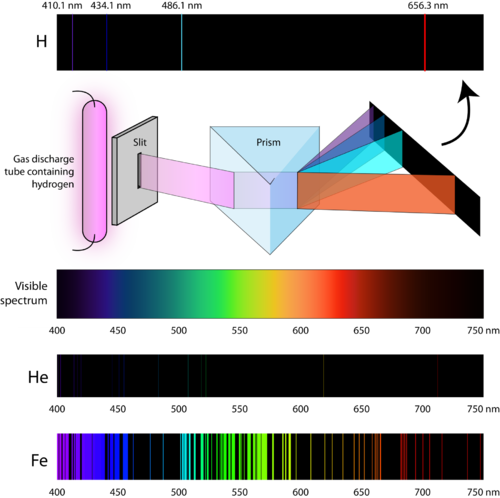
Mercury's atomic emission spectrum is shown below. What is the energy of a photon corresponding to the orange line emitted by the mercury atom. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. Emission Lines 4000-7000 Å.
Mercurys atomic emission spectrum is shown below. Thus the wavelength of the orange line is 610 nm the frequency of this emission is 492 x 10¹⁴ Hz and the energy of the emitted photon corresponding to this orange line is 326 x 10¹⁹ J. The main nitrogen emission lines and bands are evident.
101coversahugerange of wavelengths from gamma rays at 1014m to AM radio waves at 104m. The line emission atomic spectrum of an element is unique. JPEG screen grab 784 X 8.
The prominent mercury lines are at 435835 nm blue 546074 nm green and a pair at 576959 nm and 579065 nm yellow-orange. With sodium however we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum at about 589 nm. 7 Spectrum of the Hydrogen Atom.
This is not a continuous spectrum as only light of specific frequencies and specific colours are produced. Line emission spectra atomic spectra can be used to identify the presence of an element in a sample because no two elements produce the same line. A composition of a crushed rock sample was investigated using atomic emission spectroscopy.
Classical theory was unable to explain the existence of atomic emission spectra also known as line-emission spectra. Click on the name to download a text file containing an a list of emission lines in Ångstroms and their associated strengths for the corresponding element. The photon energy of the emitted photon is equal to the energy difference between the two states.
In the picture below there is the spectrum of low pressure nitrogen spectral lamp with high voltage high frequency power supply. Image and Spectrum of a Fluorescence Lamp mercury emission at 546 nm 436 nm and 405 nm and the doublet at 577 nm and 579 nm. At the right of the image are the spectral lines through a 600 linemm diffraction grating.
Based on this spectral analysis it can be concluded that the rock sample contains. The light emitted by hydrogen atoms is red because of its four characteristic lines the most intense line in its spectrum is in the red portion of the visible spectrum at 656 nm. Every element has a unique atomic emission spectrum as shown by the examples of mercury Hg and strontium Sr.
Shown below is this emission spectrum along with those of five metals. What is its frequency. Which of the given wavelengths most likely corresponds to the wavelength of the most intense line in xenons emission spectrum.
Use the Atomic Spectroscopy Lab to answer the following question. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The emission spectrum of hydrogen Lyman series.
The photograph shows part of a hydrogen discharge tube on the left and the three most easily seen lines in the visible part of the spectrum on the right. This causes line emission spectra to be produced as shown below. S 488 3 10 14 s 21.
N f 1 Unfortunately the Lyman series is not visible with the naked eye. E hf. E 6626 x 10³⁴ x 492 x 10¹⁴ E 326 x 10¹⁹ J.
11 eV 60 eV electron mercury atom Figure 9 The incident electron with kinetic energy greaterthan 49eVcollides with the mercury atom losing 49eV ofkinetic energy but retaining enough energy to be able to reach the plate. The colours of fireworks are due to it. Estimate the wavelength of the orange line.
What is its frequency. What you would see is a small part of the hydrogen emission spectrum. Most of the spectrum is invisible to the eye because it is either in the infra-red or the ultra-violet.
In the year 1885 on the basis of experimental observations Balmer proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells involved. When the atom relaxes back to a lower energy state it releases energy that is again equal to the difference in energy of the two orbits see Figure 1. Using the above information is mercury present in the Suns spectrum shown in this lab.
This formula is given as. This is caused by flaws in the way the photograph was taken. The emission spectrum of xenon is shown below.
The electromagnetic spectrum shown in Fig. Hg λ nm 400 450 500 550 600 650 700 2 615 nm 5 615 3 10 7 m n 5 cl 5 300 3 10 8 ms615 3 10 27 m 5 488 3 10 14 s 21 E photon 5 hn 5 6626 3 10 234 J. 12 mercury emission lines ranging from 254 to 597 nm sion or apparent emission lines exist that extend calibra-are suggested as useful standards with only three to four tion standards well above 600 nm into the near-infrared.
Mercury atom Figure 8 An incident electron with kinetic energy 49eVcollides with the mer-cury atom and loses all its energy. After dissolving in acid the rock sample solution was heated to produce an emission spectrum. There are two other blue lines at 404656 nm and 407781 nm and a weak line at 491604 nm.
What is the energy of an orange photon emitted by the mercury atom. There are many possible electron transitions for each atom and each. Ignore the smearing - particularly to the left of the red line.
In order to 187. The aurora borealis northern light is very rare at our latitudes and to appreciate the colours of cosmic objects powerful telescopes are necessary. It is made of all the de-excitations that end up on the n f 1 level Infinite number of them.
Emission Spectrum of Hydrogen. This series of the hydrogen emission spectrum is. N i 2 3 4.
The change in energy ΔE then translates to light of a particular frequency being emitted according to the equation E hv. In this lab we are going to be concerned with the narrow band of wavelengths 400 750 nm a nm 109m that make up visible light. Estimate the wavelength of the orange line.
Wavelengths are in the ultraviolet region-136 eV 00 eV E. Mercurys atomic emission spectrum is shown in Figure 521. H is Plancks constant 6626 x 10³⁴ Js.
This emitted light can be viewed through a spectroscope to produce a line emission spectrum a series of coloured lines on a dark background. When this light is passed through a prism as shown in the figure below four narrow bands of bright light are observed against a black background. Number of tabulated emission lines in the visible wavelength range.
Solved Figure 1 Shows The Emission Spectra Of Five Substances If You Measured Emission Spectrum Of One Of The Materials And Observed No Yellow Line Course Hero

Using Diffraction Gratings To Identify Elements Visible Spectrum Contemporary Bathroom Designs Spectrum

Atomic Emission Spectra Packer Intersections

Emission Spectrum Of The Hamamatsu 500 W Hg Xe Lamp Compared To The Sr Download Scientific Diagram
Atomic Emission Spectra Chemistry For Non Majors
Posting Komentar untuk "Mercury's Atomic Emission Spectrum Is Shown Below"