Mercury Emission Spectrum Spectrometer
We observed and recorded the different wavelengths of the spectral lines emitted by each gas. Use the Virtual Spectroscopy Lab Part 2.

An Example Of A Mercury Emission Spectrum Recorded From The Compaqs Download Scientific Diagram
In todays lab Figure 104.
Mercury emission spectrum spectrometer. Look through the grating and slit at a mercury vapor lamp. Mercury spectral calibration lamps make excellent high intensity short wave UV sources too. ConvenientThe lamp is designed so that the low pressure emissions enable quick and accurate calibrations.
The emission spectrum of a mercury discharge lamp is examined and the doublet at 576959nm and 579065nm1 due to the splitting of a single line arising from spin-orbit coupling is observed. The frequencies of these lines are inherent fundamental physical properties of mercury and do not change. The main UV emission line of ionized mercury 254 nm correlates to a blackbody of T 11500 K.
The mercury source will take at least 5 minutes warm-up. High-pressure mercury vapor and some specially-designed metal-halide lamps find application in molecular spectroscopy due to providing useful broadband continuum noise energy at millimeter and terahertz wavelengths owing to the high electron temperature of the arc plasma. The emission from a Mercury Lamp accessory measured on an Evolution 600 UV-Visible spectrophotometer Part of Thermo Fisher Scientific The emission spectrum of a mercury lamp has a number of intense lines covering the UV and visible range.
Since the lines will. Image and Spectrum of a Fluorescence Lamp mercury emission at 546 nm 436 nm and 405 nm and the doublet at 577 nm and 579 nm. To find the wavelengths of the emission lines from mercury source.
Read and record the scale positions of the brightest mercury emission lines as precisely as possible. Semrock offers a line of MaxLamp mercury line filters that are designed to isloate particular mercury lines while withstanding the intense UV radiation. In this experiment a spectrometer equipped with a diffraction grating is used to identify spe-cific wavelengths from the emission spectrum of mercury to measure these wave-lengths precisely and to compare them to accepted values.
The mercury spectrum should be set up as shown. The SL2 Mercury Argon Light Source provides accurate gas emission lines which can be utilized to verify or calibrate spectrometer wavelengths from 25365 to 101398nm. If this radiation is passed through a spectrometer a spectrum is produced.
You should see three bright lines. The Mercury Spectrum Introduction A spectrometer is an instrument used for studying electromagnetic emissions. In a previous laboratory the wavelength of.
Compare the measured spectrum with the known spectra for specific gases identify the unknown gas. Equipment Mercury discharge tube hydrogen discharge tube incandescent lamp potentiometer spectrometer with diffraction grating. To do this we used different spectrometers to look at the color spectrum that hydrogen helium mercury and nitrogen produced.
This is a reimplementation of the Emission Spectra Javascript simulation by Andrew Duffy from his collection at httpsphysicsbueduduffysimshtml. Appropriate UV eye safety measures are required. Relation to the production of light.
Emission Spectra 21Objectives measure the emission spectrum of a heated gas using the digital spectrometer. When atoms of an element are excited for instance by heating they return to their state of lowest energy by emitting radiation at specific wavelengths. Nm actual for the mercury emission line data.
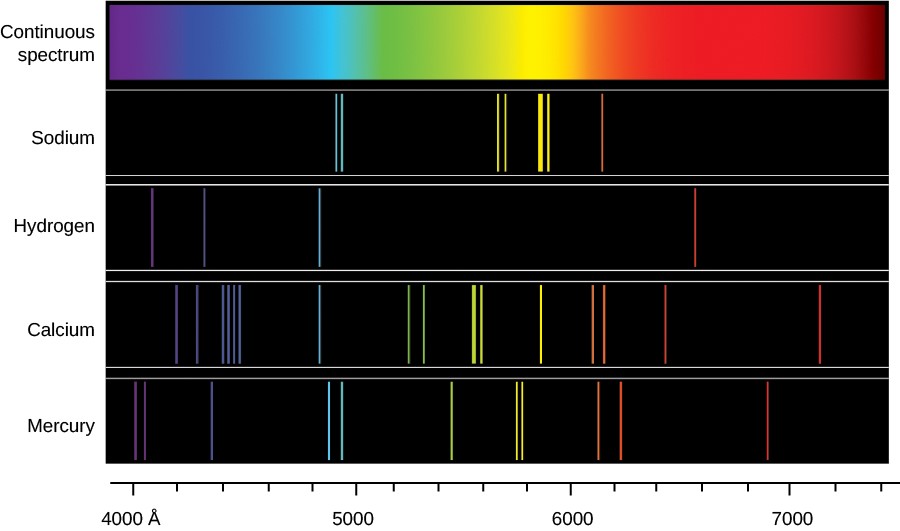
Violet 436 nm Green 546 nm Yellow 577 nm 579 nm this is actually 2 lines Red 691 nm. The wavelengths of the main lines of mercury are. Mercury light source slit optical bench mounts diffraction grating.
Model 625 PDF Data Sheet Additional Information. Emission line spectra for selected elements to observe the emission spectra for known elements and then use this information to figure out the identity of unknown elements. These ultrahigh-performance MaxLamp mercury line filters are.
SPECTROSCOPY OF LIGHT. E hc λ 1240 eV nm λ 102 where λ is the wavelength in nm and the energy of. The visible lines are intense and useful for calibration of McPherson spectrometers.
Emission spectra for hydrogen helium and mercury. Practical Laboratory 2. For this lab you will prepare an individual data sheet.
22Introduction We see very di erently than. Equation 101 can now be written as. The prominent mercury lines are at 435835 nm blue 546074 nm green and a pair at 576959 nm and 579065 nm yellow-orange.
We used this data to compare the intensity of the energy emitted from each gas. Nitrogen Spectrum In the picture below there is the spectrum of low pressure nitrogen spectral lamp with high voltage high frequency power supply. To study the spectrum of hydrogen and compare the observations to Balmers formula.
Spectrum of light from a fluorescent lamp showing prominent mercury peaks Fluorescence spectroscopy uses higher energy photons to excite a sample which will then emit lower energy photons. The emission lines of mercury are found to occur at 40421nm 40756nm 43535nm 49139nm and 54578nm. Emission spectrum of mercury.
Yellow bright green and blue. SL2 Mercury Argon Spectra. Explanation of the Emission Spectrum Max Planck presented a theoretical explanation of the spectrum of radiation emitted by an object that glows when heated.
A Mercury Spectrum Tube. More faint lines will probably be visible as well. Please write your last name and first name on this paper.
These are separated by the spectroscopes diffraction grating to produce an emission spectrum. The doublet lines are observed at 57666nm. About 90 of the total output is in the 25365nm emission line.
A labeled chart provides spectral emission lines. There are two other blue lines at 404656 nm and 407781 nm and a. Is convenient to convert the quantity hc into units of electron volts eV2.
View the mercury emission lamp through your spectroscope. At the right of the image are the spectral lines through a 600 linemm diffraction grating. Record a number of the bright lines in the spectrum.
Mercury arc lamps continue to be one of the most popular excitation sources for fluorescence microscopy because of the intense brightness they provide. Record the color and position in nm of each line that you observe on Part B of the Data SheetUsing the actual mercury transition wavelengths provided create a calibration curve of nm observed vs. This technique has become popular for its biochemical and medical applications and can be used for confocal microscopy fluorescence resonance energy transfer and.
He argued that the walls of a glowing solid could be imagined to contain a series of resonators that oscillated at different frequencies.

Wavelengths Of Spectral Lines Of Helium And Mercury Bulbs After Download Table

Plasma Radiation Spectra Of Mercury A Experimental For A Mercury Download Scientific Diagram
Zeiss Microscopy Online Campus Mercury Arc Lamps
Spectroscopy In Astronomy Astronomy

Emission Spectrum Of The Hamamatsu 500 W Hg Xe Lamp Compared To The Sr Download Scientific Diagram
Posting Komentar untuk "Mercury Emission Spectrum Spectrometer"