Mercury Emission Spectrum Wavelengths
Four more series of lines were discovered in the emission spectrum of hydrogen by searching the infrared spectrum at longer wave-lengths and the ultraviolet spectrum at shorter wavelengths. To find the wavelengths of the emission lines from mercury source.

Solved Di D2 589 6 1589 656 3 527518311517 2 1486 1 430 8 Chegg Com
Further a great deal of recent activity cence and atomic spectroscopy and is described in has extended interest to even longer wavelengths with.

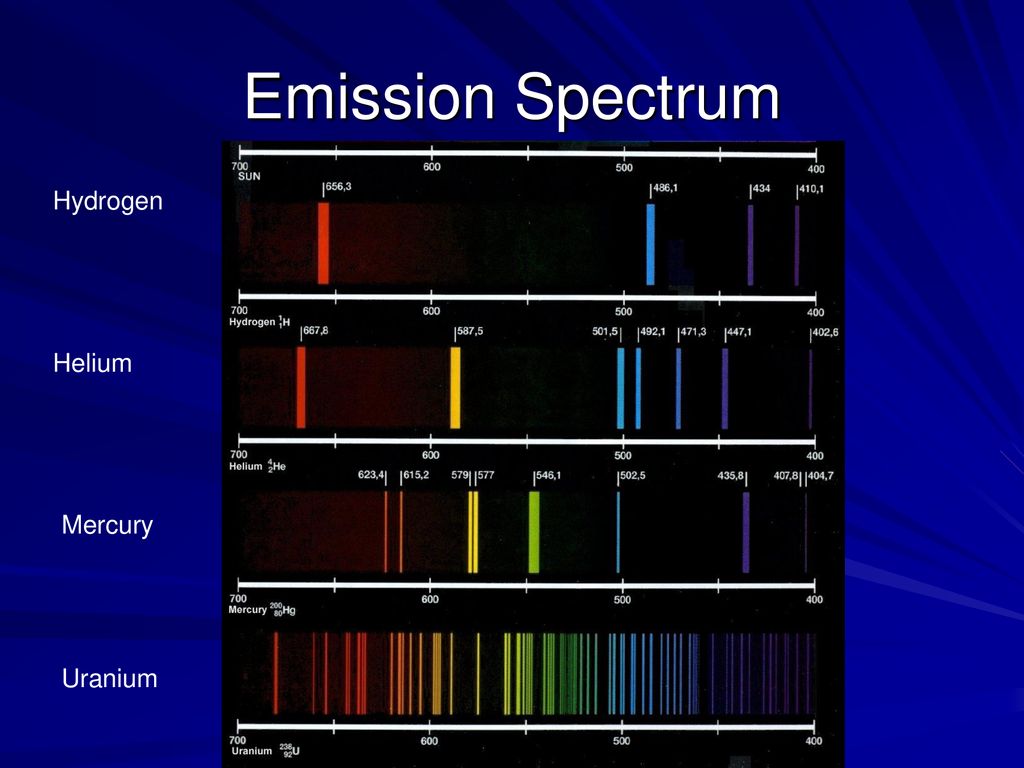
Mercury emission spectrum wavelengths. Emission spectra for hydrogen helium and mercury. Several 198Hg lines are recom-mended as secondary wavelength standards95. Each value of for the wavelength of each spectral line for the two unknown elements was within one of the accepted values.
There is a substantial number of standard spectral lines of mercury which can be produced with mercury-based spectral lamps. This light is sometimes called black light. Mercury vapor is used in the fluorescent light tubes that you see at school and home.
E hc λ 1240 eV nm λ 102 where λ is the wavelength in nm and the energy of. The frequencies of these lines are inherent fundamental physical properties of mercury and do not change. Indeed each element of the periodic table emits its own characteristic wavelengths of light.
Is convenient to convert the quantity hc into units of electron volts eV2. Typical wavelengths are 1845 nm 2537 nm 3654 nm 4047 nm 4358 nm 5461 nm 5782 nm and 1014 nm. Identi ed as mercury and helium1.
Salit and Joseph Reader The wavelengths of 19 spectral lines in the region 253 -579 nm emitted by Hg pencil -type lamps were measured by Fourier -transform spectroscopy. Wavelengths of spectral lines in mercury pencil lamps Craig J. The prominent mercury lines are at 435835 nm blue 546074 nm green and a pair at 576959 nm and 579065 nm yellow-orange.
In todays lab Figure 104. There are two other blue lines at 404656 nm and 407781 nm and a. Equation 101 can now be written as.
The strongest line at 546 nm gives mercury a greenish color. I nm 025 nm 05 nm I nm 2 an and 3. The collection of the different distinct wavelengths emitted by an atom is called the emission spectrum of the atom.
The main UV emission line of ionized mercury 254 nm correlates to a blackbody of T 11500 K. The emission spectrum of a mercury lamp has a number of intense lines covering the UV and visible range. At left is a helium spectral tube excited by means of a 5000 volt transformer.
The mercury emission line at 4358 nm was scanned for spectral bandwidths of 0. High-pressure mercury vapor and some specially-designed metal-halide lamps find application in molecular spectroscopy due to providing useful broadband continuum noise energy at millimeter and terahertz wavelengths owing to the high electron temperature of the arc plasma. The emitted light is not very bright for just the mercury vapor but when scientists examined the full spectrum for mercury they saw what you just observed.
At the right of the image are the spectral lines through a 600 linemm diffraction grating. In low-pressure mercury-vapor lamps only the lines at 184 nm and 253 nm are present. Precise calibration of the spectra was obtained with wavelengths of 198 Hg as external standards.
To find wavelengths of prominent spectral lines of Mercury by Diffraction Grating Physics Practical. The visible yellow light has a wavelength of about 570 nm. In this experiment a spectrometer equipped with a diffraction grating is used to identify spe-cific wavelengths from the emission spectrum of mercury to measure these wave-lengths precisely and to compare them to accepted values.
Mercury light source slit optical bench mounts diffraction grating. The standard deviation Hg in the values of for mercury was Hg 611 nm and the standard deviation H for hydrogen was H 115 nm. Only the light at 253 nm is usable unless synthetic quartz is used to manufacture the tube as the line is otherwise absorbed.
Wavelengths nm 438793 w. The compact DUV RF spectrometer has a spectral range from 2499 nm to 3536 nm 200 cm-1 to 12000 cmâ 1 with a spectral resolution of 0296 nm â ¼40 cmâ 1. Each of these lines fits the same general equation where n 1 and n 2 are integers and R H is 109678 x 10 -2 nm.
Therefore mercury lamps are primary standards and do not require recalibration making them a dependable economical solution for ensuring. Mercurys predominant lines right to left are at 5791 5770 angstroms yellow 5461 green 4916 cyan. The Mercury Spectrum Introduction A spectrometer is an instrument used for studying electromagnetic emissions.
LED UV lights have a narrow spectral output centered around a specific wavelength 10nm. Green is reflected therefore grass appears green. Emission spectrum of mercury.
Grass for example appears green because all of the colors in the visible part of the spectrum are absorbed into the leaves of the grass except green. The mercury spectrum has been studied for many years. 232 Confirmation of Spectral Bandwidths The mercury line source was used to confirm the spectral bandwidth settings of the spectrophotometer.
To use a spectroscope to determine the wavelengths of emission lines of hydrogen helium mercury and nitrogen. A spectral line is like a fingerprint that can be used to identify the atoms elements or molecules. At the right of the image are the spectral lines through a 600 linemm diffraction grating.
It is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency. In medium-pressure mercury-vapor lamps the lines from 200600 nm are present. The strongest peaks of the emission line spectrum are.
The pastel color of argon is due to a wide range of lines throughout the spectrum. The use of mercury vapor lamps as wavelength cali- emission of many fluorophores into the midvisible or red bration standards is a common practice in both fluores- spectral regions. There is an enormous emission in the ultraviolet region UV.
If this radiation is passed through a spectrometer a spectrum is produced. Mercury lamps are convenient sources for wavelength calibration lines and are widely used to calibrate spectrometers. The light from the mercury discharge tube was composed of only three colors or three distinct wavelengths of light.
When atoms of an element are excited for instance by heating they return to their state of lowest energy by emitting radiation at specific wavelengths. A list of 92 lines claimed as due to mercury was published by Kayser and Runge1 in 1891.

Pdf Wavelengths Of Spectral Lines In Mercury Pencil Lamps Semantic Scholar

The Emission Spectrum Of The Mercury Light Bulb Download Scientific Diagram

Second Order Spectra Vs First Order Physics Stack Exchange
Atomic Emission Spectra Ppt Download

Wavelengths Of Spectral Lines Of Helium And Mercury Bulbs After Download Table
Posting Komentar untuk "Mercury Emission Spectrum Wavelengths"