Mercury Vapor Emission Spectrum
Metal halide lamps. This light is sometimes called black light.
Zeiss Microscopy Online Campus Mercury Arc Lamps
What amount of energy in joules must be released by an electron in a mercury.
Mercury vapor emission spectrum. Spectral lines which are often used as wavelength references. The lines result from emissions of those colors by excited mercury atoms. The emitted light is not very bright for just the mercury vapor but when scientists examined the full spectrum for mercury they saw what you just observed.
The lamps had internal diameters of 5 and. In contrast the mercury lamp spectral emission region between 600 and 1000 nanometers is relatively continuous and no brighter in output than xenon arc lamps which span a broad spectral range with only a few spectral lines in the blue and infrared regions. The yield is 80-100 lumens per watt and the life is about 10000 hours.
At the right of the image are the spectral lines through a 600 linemm diffraction grating. A spectrophotometer uses the mercury emission lines to calibrate the displayed wavelength values. Mercury vapor lamps are metal vapor lamps based on light emission from mercury Hg atoms.
LP and MP mercury lamps differ in terms of mercury vapor pressure emission spectrum UV flux wall temperature lifespan etc. There is an enormous emission in the ultraviolet region UV. Line spectra are produced by atomic substances such as sodium in a sodium vapor lamp mercury in a mercury vapor lamp and gases in a discharge tube.
The yield is 30-60 lumens per watt and the life is about 20000 hours. No light is emitted with those wavelength values. The emission spectra of four mercury vapor lamps are used to obtain wavelength calibration curves for the double-grating emission monochromator of a spectrofluorimeter.
Atoms in the gaseous state also known as free excited atoms produce a line spectrum. The light produced by white LEDs has a color temperature spectrum similar to that of a mercury vapor lamp which is in the daylight illumination category. The spectral radiance of the 185 and 254 nm lines in two positive column mercury discharge lamps was measured over a wide range of operating conditions.
The maximum emission moves from the 2537nm resonance line to the 365nm 3 P-3 D non-resonance line. Mercury Hg is the only elementary metal which has a substantial vapor pressure at room temperature. The strongest peaks of the emission line spectrum are In low-pressure mercury-vapor lamps only the lines at 184 nm and 253 nm are present.
Therefore a lamp enclosing may in principle contain mercury only but often there is. A bright violet line occurs at 4358 nm in the emission spectrum of mercury vapor. The strongest peaks of the emission line spectrum are.
Its used to figure out what kind of gas youre dealing with. They are also of the gaseous type. When the pressure in a mercury vapor discharge increases the spectrum of the mercury discharge is altered significantly.
This UV radiation is then converted into visible white light by UV excitation of a fluorescent coating on the inside of the glass tube. Emission spectrum for mercury vapor. LP mercury lamps operate at a pressure of approximately 1 Pa 001 mbar and emit UV radiations at 2537 and 1850 nm Schalk et al 2006 former being absorbed by pathogens disrupting their DNA sequences while the latter is applied in advanced oxidation processes AOPs.
By the electrodeless method with temperatures ranging from 160C to over 200C and. These lamps have an orange yellow tint. Mercury-vapor Lamp - Operation - Emission Line Spectrum.
Mercury vapor is used in the fluorescent light tubes that you see at school and home. The 254 nm 365 nm 436 nm or 546 nm emission lines can be used for the calibration but care is required with the slit width spectral bandwidth used during measurements. As the main arc strikes and the gas heats up and increases in pressure the light shifts into the visable range and the high gas pressure causes the mercury emission bands to broaden somewhat producing a light that appears more nearly white to the human eye although it is still not a contenious spectrum Even at full intensity the light from a mercury vapor lamp with no phosphors is distinctly bluish in color.
The 2537nm line becomes greatly suppressed and approximately half the energy is now radiated at the 365nm line. Here we use a diffraction gradient to observe the visible spectrum of hydrogen helium mercury vapor and neon. Examining the white LED emission spectrum presented in Figure 3 the transmission peak at 460 nanometers is due to blue light emitted by the gallium nitride diode semiconductor while the.
You can see from the emission spectrum why the sodium vapor lamps would appear yellow and not white. 2 when the same tube in an electric oven was excited to luminosity by a high frequency electrodeless discharge. In comparison mercury vapor lamps produce a bluecolored light due to an intense light emission from the mercury atoms in the visible part of theelectromagnetic EM spectrum.
The black areas are wavelengths that are not emitted by mercury. The visible light emitted by mercury are the ten colors shown here. The continuous band spectrum of mercury vapor was observed 1 when a drop of mercury-was kept rolling in a heated cylindrical quartz tube by means of a horizontal to and fro motion of the tube.
The compact DUV RF spectrometer has a spectral range from 2499 nm to 3536 nm 200 cm-1 to 12000 cmâ 1 with a spectral resolution of 0296 nm â ¼40 cmâ 1. Only the light at 253 nm is usable unless synthetic quartz is used to manufacture the tube as the line is otherwise absorbed. This explains why the light produced from sodium vaporlamps would appear yellow and not white.
They also pass electricity through a gas. A fluorescent lamp generates light from collisions in a hot gas plasma usually containing mercury which emit photons at two UV emission lines - 254nm and 185 nm. In low-pressure mercury-vapor lamps only the lines at 184 nm and 253 nm are present.
It is a property of the emitting substance. The use of second- and third-order diffraction lines and emission lines from the argon carrier gas provides a rich spectrum. These lamps consume less energy than the older blue colored mercury vapor lamps.

Emission Spectrum Of A Mercury Vapor Lamp Download Scientific Diagram
Materi Tik Ptd Lampu Elektrik Electrical Discharge Lamps
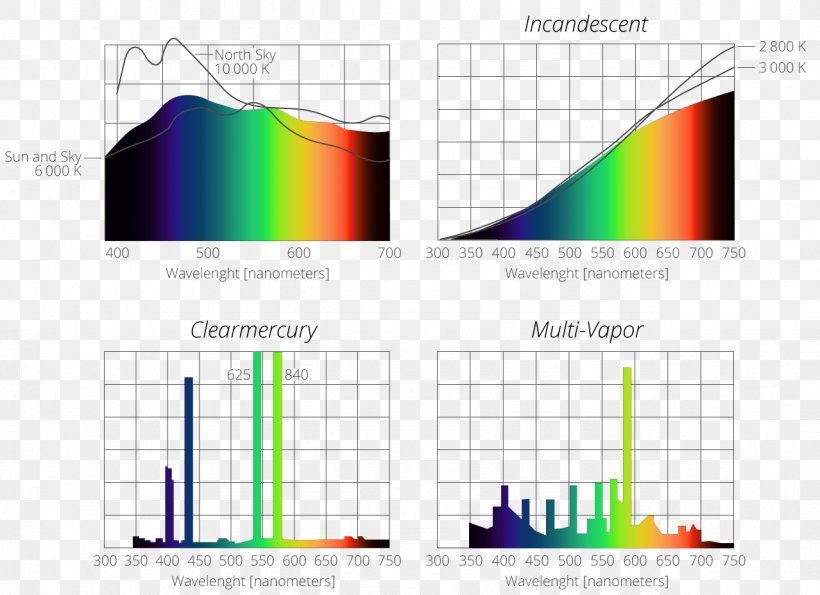
Incandescent Light Bulb Emission Spectrum Mercury Vapor Lamp Png 1278x928px Light Area Diagram Electromagnetic Spectrum Elevation

Emission Spectrum Of The Employed Low Pressure Mercury Vapor Lamp Download Scientific Diagram
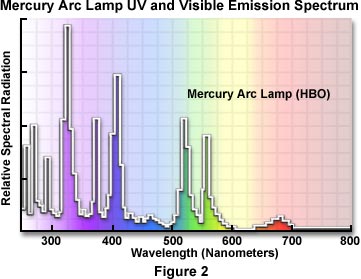
Posting Komentar untuk "Mercury Vapor Emission Spectrum"