Mercury Ii Nitrate Ammonium Sulfide Balanced Equation
Submit Request Answer Type here to search 1150 AM 1202020. Lithium lead IV phosphate 5.
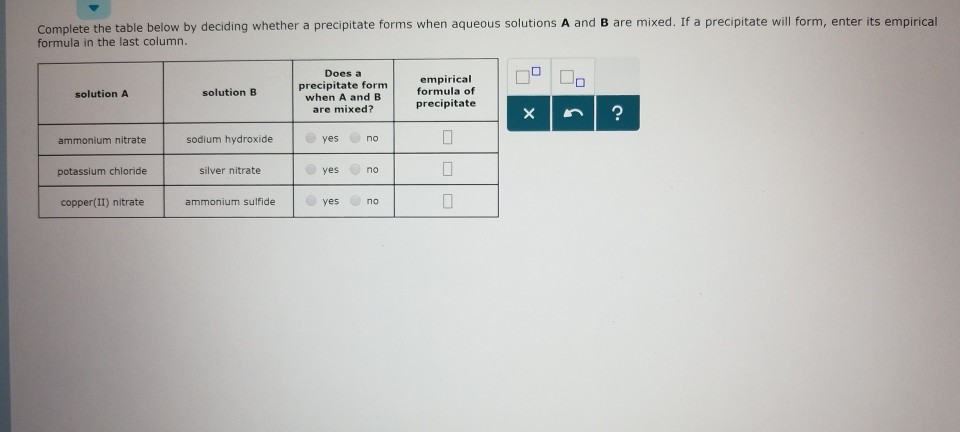
Solved Complete The Table Below By Deciding Whether A Chegg Com
Split the reaction into two half reactions Step 2.

Mercury ii nitrate ammonium sulfide balanced equation. 1 Sulfur trioxide reacts with water forming sulfuric acid. More Practice - Predicting and Balancing Equations Worksheet. Solutions of manganeseII sulfate and ammonium sulfide are mixed.
MercuryII nitrate ammonium sulfide mercuryII sulfide ammonium nitrate 2 HgNO 3 2 NH 4 2 S HgS 2 NH 4 NO 3 5. Barium water 2HLO 4. Ammonium sulfide and lead II nitrate.
Water hydrogen oxygen 4 2 H 2 O 2 H 2 O 2 7. Iron Ill Sulfate Calcium Nitrate 8. Calcium mercury 1 chloride CA 7.
Prolonged exposure to fireside or heat may end in an explosion. Barium Iodide Sodium Sulfate 3. 4mercuryII nitrate ammonium sulfide mercuryII sulfide ammonium nitrate 2 HgNO 3 2 NH 4 2 S HgS 2 NH 4 NO 3 5ironIII hydroxide ironIII oxide water 5.
Copper Silver nitrate Copper II nitrate Silver 3. PbNO32 aq Pb2 aq 2NO 3 aq In order to precipitate the lead II cations hydrogen sulfide H2S is bubbled through the solution. Multiply the half reactions in order to balance electrons lost and electrons gained.
Zinc chloride ammonium sulfide zinc copper II sulfate magnesium bromide chlorine aluminum oxide silver nitrate sodium chloride magnesium copper II nitrate sodium hydroxide sulfuric acid lead II nitrate potassium bromide. Write a balanced chemical equation for each of the following reacions. Ammonium sulfide leftmathrmNH_4right_2 mathrmS.
Write a balanced equation for each of the following decomposition reactions. Aluminum Iodine I O. Magnesium Hydroxide Ammonium Sulfate.
Write the equation and net ionic equations for the following reactions. In the balanced chemical equations what is the ratio of coefficents Submit Request Answer Part B - Identity the precipitate IV. WORKSHEET 8 Write the balanced chemical equations for the following 1.
Water Hydrogen Oxygen 7. Lead Il nitrate potassium chromate 8. Translate the following sentences that each describe a chemical reaction into balanced chemical equations.
Mercury II Nitrate is a white crystalline solid. A balanced equation has equal numbers of each type of atom on each side of the equationBalanced chemical equations mercury sulfide plus ammonium nitrate is as follows Hg2SO4 2. A piece of aluminum metal is added to a solution of silver nitrate.
2O H 2O 2KOH 16. Need to follow the given equation enter either the number of moles or weight for one the. Copper Il chloride and lead Il nitrate solutions are mixed together.
Sodium Water -Z 9. A balanced equation has equal numbers of each type of atom on each side of the equationBalanced chemical equations mercury sulfide plus ammonium nitrate is as follows Hg2 SO4 2 NH4 NO3. Iron III hydroxide Iron III oxide water 6.
CopperII nitrate water hydrogen nitrate copperII hydroxide 4 CuNO 3 2 2 H. Mercury II Nitrate Formula. Aluminum chromic acid Y 2.
Ammonium sulfide solution is poured in an aqueous cadmium nitrate solution. Iron Ill Hydroxide Hydrobromic Acid 11. Silver acetate gold Ill chloride 3 6.
Iron Ill hydroxide hydrobromic acid 3. Ha 14 co silver nitrate potassium chloride iron Il sulfide hydrochloric acid 9 FeCz Fests ammonium nitrate sodium acetate Rx. Balancing Word Equations 1.
A Potassium hydrogen carbonate decomposes by heating to give solid potassium carbonate water and carbon dioxide gas. Balance each half reaction the ones shown above are not balanced Step 3. Complete balanced equation below it.
Iron III sulfate and barium iodide. Potassium hydroxide phosphoric acid potassium phosphate water 3KOH H 3PO 4 K 3PO. I sulfide ammonium sulfide mercury II nitrate ammonium nitrate no reaction silver copper.
Clearly show reactants products and coefficients when applicable. Magnesium sulfuric acid. 2 Zinc and sulfur combine to form zinc sulfide.
MercuryII Nitrate HgCl2 MercuryII Chloride HgO MercuryII Oxide HgS MercuryII Sulfide HNO2 Nitrous Acid HNO3 Nitric Acid K2C2O4 Potassium Oxalate K2CO3 Potassium Carbonate K2Cr2O7 Potassium Dichromate K2CrO4 Potassium Chromate K2HPO4 Potassium Hydrogen Phosphate K2O Potassium Oxide K2S Potassium Sulfide K2SO4 Potassium Sulfate K3PO4. Write balanced formula equations for these reactions and identi6r the type of reaction. II sulfate and mercuryI nitrate react to give a mercuryI sulfate.
Constants Part A - An aqueous solution of manganese II nitrate is combined with an aqueous solution of ammonium sulfide. It produces toxic oxides of nitrogen when heated to decomposition. Balance the equations using symbols g aq l s when appropriate.
Mercury II hydroxide phosphoric acid mercury II phosphate water 3HgOH 2 2H 3PO 4 Hg 3PO 4 2 6H 2O 18. IronIII hydroxide ironIII oxide water 5 2 FeOH 3 Fe 2 O 3 3 H 2 O 6. Zinc Cupric sulfate Zinc sulfate Copper 4.
Aluminum metal is added to a solution of copperII chloride. Solutions of zinc nitrate and calcium sulfide are mixed. Excess hydrochloric acid solution is added to a solution of potassium sulfite.
16H 2MnO 4- 10Cl-2Mn 2 5Cl 2 8H 2 O Here are the general steps in balancing a redox reaction. Phosphoric Acid Magnesium H 7. The complete ionic equation.
If no reaction occurs simply write only NR. The molar mass of mercuryII nitrate is 32460 gmol. 46 O of 1 point earned 3 attempts remaining Write the balanced NET ionic equation for the reaction when mercury 1 nitrate and ammonium sulfide are mixed in aqueous solution.
Classify the reation type. Potassium Iodine 2. Cl-Cl 2 The overall equation is.
Zinc chloride ammonium sulfide ZACIZK SQ 2 mercury Il sulfate ammonium nitrate cobalt Ill hydroxide nitric acid phosphoric acid sodium hydroxide. Mercury II nitrate Ammonium sulfide Mercury II sulfide Ammonium nitrate 5. A balanced equation has equal numbers of each type of atom on each side of the equationBalanced chemical equations mercury sulfide plus ammonium nitrate is as follows Hg2SO4.
Carbon Oxygen Carbon dioxide 2. Type of chemical reaction was a single displacement reaction one copper silver nitrate word equation. The density of it is 43 gcm3.
This will result in the formation of lead II sulfide PbS an insoluble ionic compound that will precipitate out of solution. Ammonium sulfide lead II nitrate ammonium nitrate lead II sulfide NH 4 2S PbNO 3 2 2NH 4NO 3 PbS 17. Use yo ts- solubility rules 26.

How To Balance Pb No3 2 Nh4oh Nh4no3 Pb Oh 2 Youtube

Metathesis Reactions Example Aluminum Sulfate And Ammonium Nitrate Youtube

Printable Balancing Chemical Equations Worksheet Chemical Equation Equations Balancing Equations
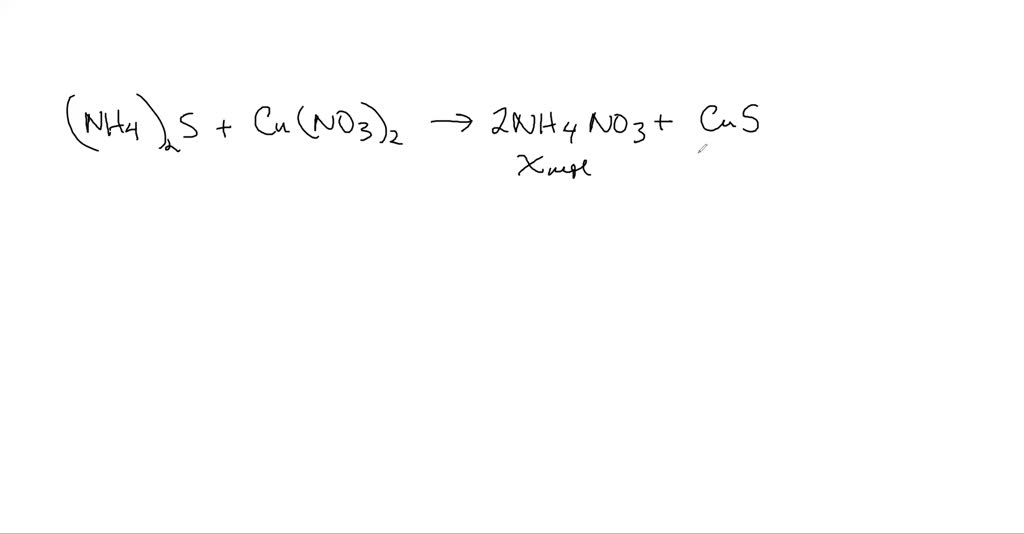
Solved Ammonium Sulfide Reacts With Copper Ii Nitrate In A Double Replacement Reaction What Mole Ratio Would You Use To Determine The Moles Of Mathrm Nh 4 Mathrm No 3 Produced If The Moles Of Cus Are Known

Solved 6 80 Ml Of A 1 30 M Solution Of Lead Ii Nitrate Was Chegg Com
Posting Komentar untuk "Mercury Ii Nitrate Ammonium Sulfide Balanced Equation"